New materials are constantly being developed to make our lives easier, safer, and healthier. from new types of plastics to carbon fiber, kevlar, and self-cleaning fabrics, These new materials show how chemistry can create new industries and transform the world. They even allow athletes without lower legs to compete in running events at the Paralympic and Olympic games.
Social needs
The development, manufacturing and sale of new materials provide work and income. New materials can give a company an advantage, allowing it to make more money.
Ethical needs
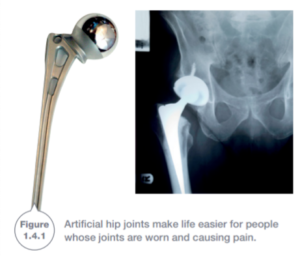
Ethics is an assessment of whether it is right or wrong to do something. Developing new materials because they make peoples lives healthier or safer is obviously the right thing to do. For example, metals have been developed for artificial limbs and artificial joints.
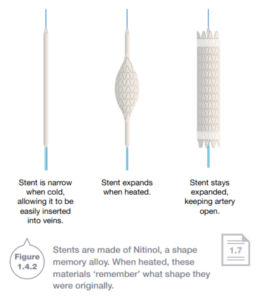
Shape memory alloys such as Nitinol change shape as temperature changes. nitinol is used to construct small mesh sleeves called stents, which are used to keep open arteries that are in danger of bursting or blocking. The stent is cooled and crushed to make it thin enough to be surgically inserted into a vein in the groin. The stent is then pushed through the veins to the heart. As the body hearts the alloy, the nitinol in the stent ‘remembers’ its original expanded state and opens up to widen the artery.
Environmental needs
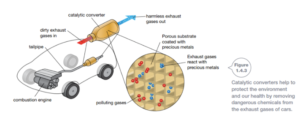
New materials are also developed because they are better for the environment. For example, the metal rhodium is used in catalytic converters in car exhaust systems. Catalytic converters remove poisonous chemicals from the exhaust, making the air cleaner and healthier for people living near busy roads. Likewise, the development of biodegradable plastics that are able to decompose ensures a cleaner environment.
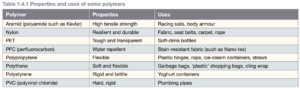
Polymers and synthetic fibers
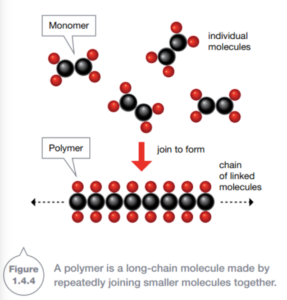
Polymers are molecules made of repeating units, called monomers, formed into long chains.
Naturally occurring polymers include cellulose, proteins, starch, DNA, and rubber. Plastics are synthetic polymers.
Fiber is any substance that can be twisted into a rope, woven or knitted into a fabric, or mixed with other substances to form a mesh. Fibers are solid with molecules arranged in long chains that are tangled together but not necessarily chemically bonded together.
Self-cleaning fabric
Self-cleaning fabrics are coated in molecules of a polymer such as PFC (per fluorocarbon). These fibers hold water droplets away from the surface of the fabric, preventing water from sticking to it. This makes the fabric hydrophobic.
A surface is classified as hydrophobic if it does not allow water to stick to it. A hydrophobic surface is highly water repellent and is commonly referred to as ‘water-hating’. Whereas hydrophilic means ‘water-loving’.

Carbon fiber
A carbon nano tube is a tiny cylinder of carbon atoms about 100 nano-meters long. A nano meter is one-billionth of a meter. About 25 nano tubes would fit across a red blood cell.
Carbon atoms can join with each other to form flat sheets of hexagons. These tubes can be formed into fibers and are incredibly strong. For this reason, nano-tubes are being researched for use in the structures of cars, aircraft, and buildings. Carbon nano-tubes are also being researched for possible use in electronic devices such as semiconductors and microprocessors.

- List four examples of:
- natural polymers
- synthetic polymers
- Name a self-cleaning fabric
- State the length of a nanometre
- State two advantages of carbon nanotubes over steel
- Define the term polymer
- Correct the following statements to make them correct
- Polymers are long-chain synthetic compounds made by scientists
- The contact angles on super-hydrophobic surfaces are 90˚C
- Explain what is meant by a fibre
- Compare the properties of PVC and polythene and relate them to their uses
- Contrast the terms hydrophobic and hydrophili.

