The 118 elements of the periodic table are classified as metals, non-metals, or metalloids. These are used in very different ways. Metals are used to make electrical wiring for ships, nails, and saucepans. Non-metals are used to make plastics fertilizers, antiseptics, and fuels, while metalloids are used to construct electronic chips for iPods and laptops.

Elements
- Elements are substances that are made up of only one type of atom. Each atom in an element has the same number of protons in its nucleus.
The periodic table
There are 118 different elements and therefore 118 different types of basic atoms. The periodic table is a list of all 118 known elements, arranged in order of their atomic number.
Metals
Properties of metals:
- Metals are lustrous (they shine when polished)
- malleable (They can be bent into new shapes without breaking)
- ductile (they can be stretched into wires)

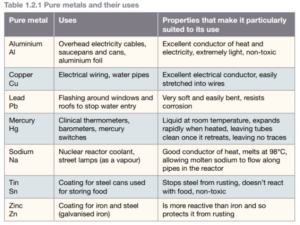
Pure metals
Most metals cannot be used as pure elements because they have propertes that make them impractical. For example, most pure metals are too soft to be made into anything useful.
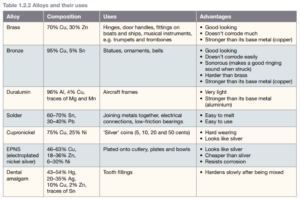
Alloys
Most of the metals around you are not pure elements but alloys. An alloy is a metal combined with small amounts of other elements.
An example of this steel is much stronger and harder than its iron base metals, allowing it to be used in everything from paperclips, staples, nails, and screws to cars, ship hulls, and the frames of bridges and skyscrapers.
Steel is an alloy of iron with small amounts of carbon added to it. Different amounts of carbon produce different steel alloys.
- Wrought iron contains almost no carbon and is the closest alloy to pure iron.
- Mild steel has only 0.5% carbon
- hard steel or tool steel has about 1% carbon
- Cast iron has been 2.4% and 4.5% carbon. Cast iron is strong but brittle, shattering easily if dropped or hit.
Steel can be further improved by adding chromium and nickel to it. This creates rust-resistant stainless steel. Stainless steel is used in hot, wet, and salty environments that would cause rapid rusting of other types of steel. This is why stainless steel is used in kitchens, on ships, for surgical instruments, and for jewelry for body piercings.

Pure gold is so soft and fragile that any jewelry made from it would soon break. For this reason, silver and/or copper are added to it to create a stronger alloy. The carat scale measures the amount of pure gold in jewelry, with pure gold rated as 24 carats. Jewelry is often 18 carat, meaning that is 18/24 (75%) Gold.
Non-metals
Most non-metals are found naturally as gas in the air. A few are solids found in the Earth’s crust, such as the sulfur that occurs around volcanoes. The physical properties of non-metals are very different from those of metals.

Carbon
Carbon is an unusual element because its atoms combine with other carbon atoms of other elements (usually hydrogen and oxygen) to form lattices, long chains, and rings. Over 90% of all known compounds contain carbon.
Pure carbon exists in several different forms, called allotropes. Three common allotropes are:
- Amorphous carbon
- Diamond
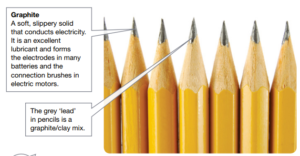
- Graphite
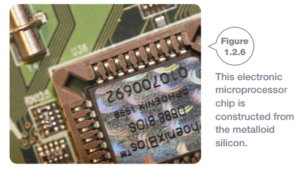
Metalloids
Metalloids (sometimes called semi-metals) act as like non-metals in most ways. However, they also have some properties similar to metals.