The soluble substances in a solution are far too small to settle or be trapped in filter papers. Different methods are therefore needed to separate them from the solvent they are dissolved in. In the photo a separation method called distillation is being used to make perfume from rose petals.
Chromatography
Recall:
- A mixture contains many different substances
- Solutes dissolve in solvents
- Not all substances can dissolve in water.
- Even if something does dissolve, it may not dissolve as well as a different substance.
Chromatography is a process that can separate a mixture by making it move through another substance such as a gel, column of liquid or strip of paper, as shown in Figure 4.3.1. Here, water is the solvent. It dissolves the dyes from ink or food dyes and carries the colours with it as it moves up the paper.
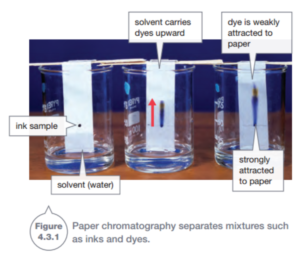
Chromatography works because different chemicals in mixtures are attracted to the paper by different amounts. Substances that are strongly attracted to the paper are harder for the solvent to move along. These substances do not move very far. Weakly attracted substances move the furthest.
Chromatography is very important in industry. It is used to find out what is in oil and gas, and to identify pollutants.
Chromatography is also used by pharmaceutical manufacturers to analyse plants and animals for possible useful medical drugs and to test the quality of their products. Environmental scientists use chromatography to identify chemical pollutants.
Evaporation
Evaporation is the change in state when a liquid becomes a gas. Water boils at 100˚C but it does not need to be 100˚C to evaporate. Water evaporates at all temperatures above 0˚C, this means that wet clothes would try at any temperature above 0ºC. However evaporation speeds up the higher the temperature. This is why clothes dry faster on a hot day instead of a cold one.
If the water has any solute dissolved in it, then evaporation will leave that solute behind. For example, sea water is an aqueous solution of salt dissolved in water. After swimming in the sea, the water on your skin evaporates, leaving a thin layer of salt behind.
Evaporation is commonly used in the laboratory to separate a solvent from its solute.The solution can be left in the air to evaporate using the heat of the room, but a Bunsen burner or hotplate speeds the process up.
Figure 4.3.2 shows how this can be done. The solute is left behind in the evaporating dish, while the solvent (usually water) is lost to the air. This means that you can only collect the solute.

Crystallisation
The solute left behind by evaporation forms crystals.
You can see some different crystals in Figure 4.3.3. Crystals have distinctive shapes because the solute particles lock into one another like pieces of a jigsaw. As the solvent evaporates, the solution becomes more and more concentrated. (Cooks commonly use evaporation to concentrate the flavors of their sauces.) Eventually the solution becomes so concentrated that it is saturated.The solute particles start to lock in with one another, and the crystals grow as more of the solvent evaporates. This process is called crystallization.
Smaller crystals form when the solvent evaporates quickly. In contrast, larger crystals form when the solvent evaporates slowly.
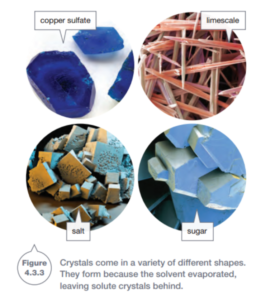
Evaporation and crystallization are used in industry to remove soluble substances from solutions and to purify substances. For example, salt producers make salt by using the heat from the Sun to evaporate water from pools of salt water. This leaves crystals of salt behind to be collected (Figure 4.3.4).

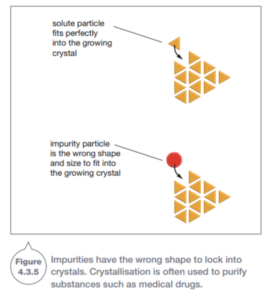
Crystallization is also used in industry to purify substances such as pharmaceuticals (medical drugs) A solution may have unwanted substances dissolved in it as well as substances that are wanted. The unwanted substances are called impurities.
The particles of impurities generally do not have the same shape as the solute particles. So if you crystallize the solute, the impurities generally will not have the right shape to lock into the growing crystals (Figure 4.3.5). Instead, the impurities stay in solution and the crystals formed stay pure.
Distillation
Evaporation is the process in which a liquid turns into a gas. Condensation is the opposite: a gas cools to form a liquid. Distillation uses both evaporation and condensation to separate substances.
Evaporation loses the liquid solvent to the atmosphere, but sometimes you need to keep the liquid as well. In distillation, the gas is condensed back into a liquid so that it can be collected. If the solvent is water, distillation first evaporates off the water. It then cools the water vapor so that it condenses back into liquid water. The apparatus that converts the gas back to the liquid is called the condenser. Figure 4.3.6 shows a special apparatus called a Liebig condenser that is often used in the laboratory.

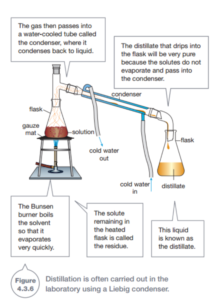
Distillation is able to separate several liquids from each other if they have different boiling points. For example, alcohol has a boiling point of78°C while water boils at 100°c. These two liquids can therefore be separated by distillation. All of the alcohol will evaporate from the mixture first (at 78°C), leaving the water behind. The water will then evaporate off at 100°c, leaving behind whatever solute was dissolved in it.
As well as separating solutions in laboratories, distillation is used in:
- producing alcoholic drinks such as vodka and bourbon
- separating crude oil into petrol, diesel, lubricating oils and other components
- removing impurities from drinking water
- separating oxygen, nitrogen and argon from air for industrial use
- perfume manufacture
4.3 Unit review





