Each of the states of matter has its own characteristic properties that can be explained using a simple model called the particle model.
Models in science
Scientists use models to test or explain something that is difficult to understand. The model like the particle model is used to explain how and why states of matter behave the way they do.
There are 2 main types of models in science:
- Analogies: The heart is often compared with a water pump. This simple type of model is known as an ‘analogy’. An analogy uses a common, everyday thing to help us understand how something that is complicated works. Similarly, a computer is sometimes used as an analogy for the brain.
- Thought models: Models can also be ‘thought’ models. Thoughts models help scientists imagine objects and events that are difficult to understand. Such as for incredibly large objects or events like the universe.

The particle model
The particle model is a ‘thought’ model that attempts to explain the properties of substances.
In the particle model, all substances are thought to be made of incredibly small, hard, spherical balls called particles.
Each ball has energy and moves according to how much energy it has. If the particle has lots of energy, then it will move about a lot. If the particle has very little energy, then it will be sluggish and move about slowly.
You add energy to a substance whenever you heat it. This causes a particle to move about more, and faster.
If you cool a substance then the reverse will happen, the particles move about less and move more slowly.
the particle model assumes the following:
-
All substances are made up of tiny, hard particles that are too small to see even with a normal microscope.
-
The particles always have energy and are moving.
-
The particles move about more and move faster as the temperature is increases.
-
the closer the particles are to one another, the stronger the attraction between them.
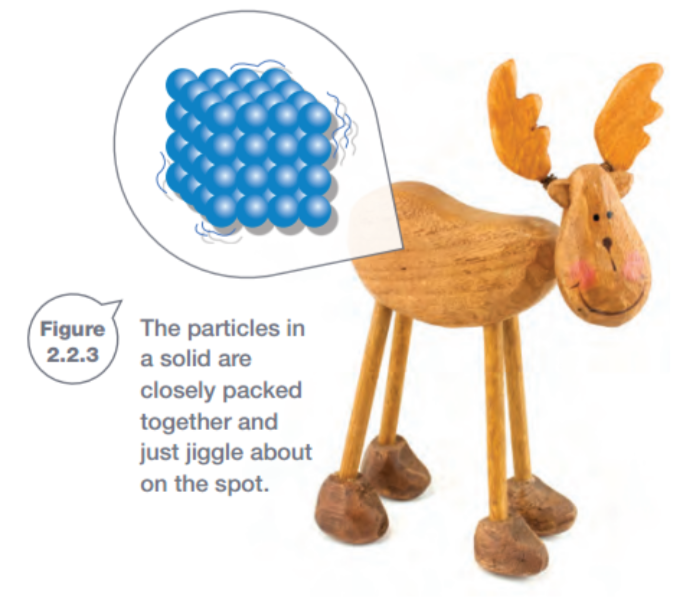
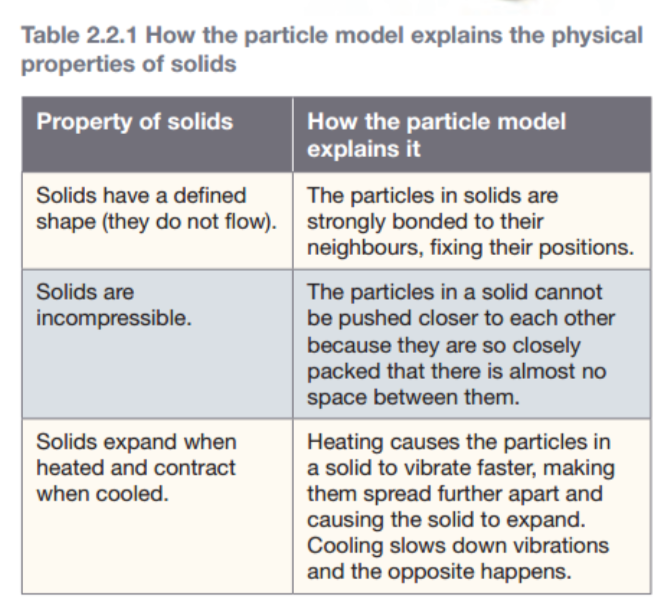
Explaining solids
In solids, the particles are closely packed in fixed positions. Forces between particles that are next to each other form bonds (joins), that hold all the particles in the solid closely together. The particles don’t break out of position and instead just vibrate about on the spot.
Explaining liquids
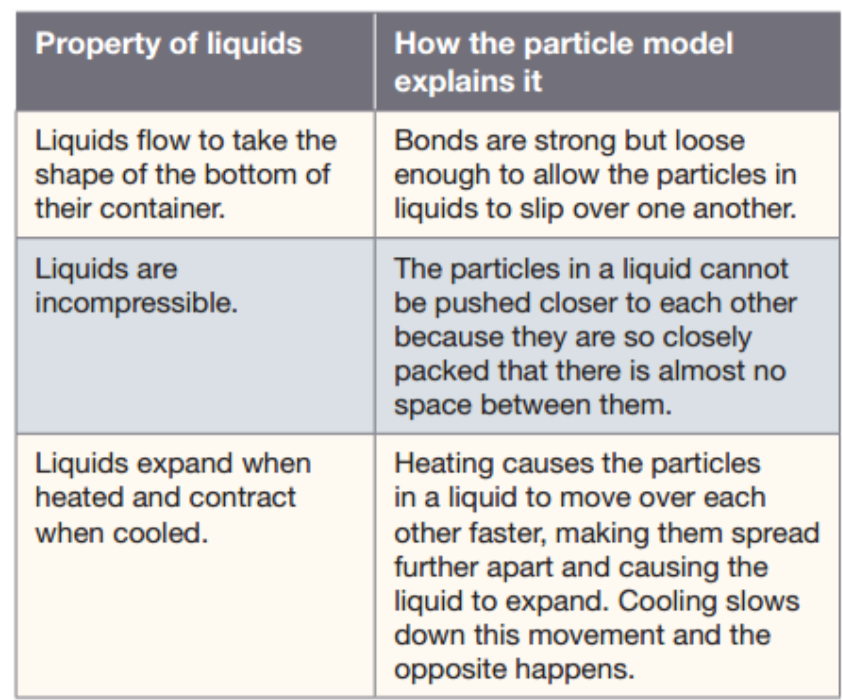
In liquids, the particles are still packed closely together but they are far more loosely bonded (joined) to their neighbors than the particles are in a solid.
The loose binding allows the particles to move about over each other, allowing the liquid to flow, drip, and fill the bottom of whatever container it is in. As the liquid is heated, this movement is faster.

Explaining gases
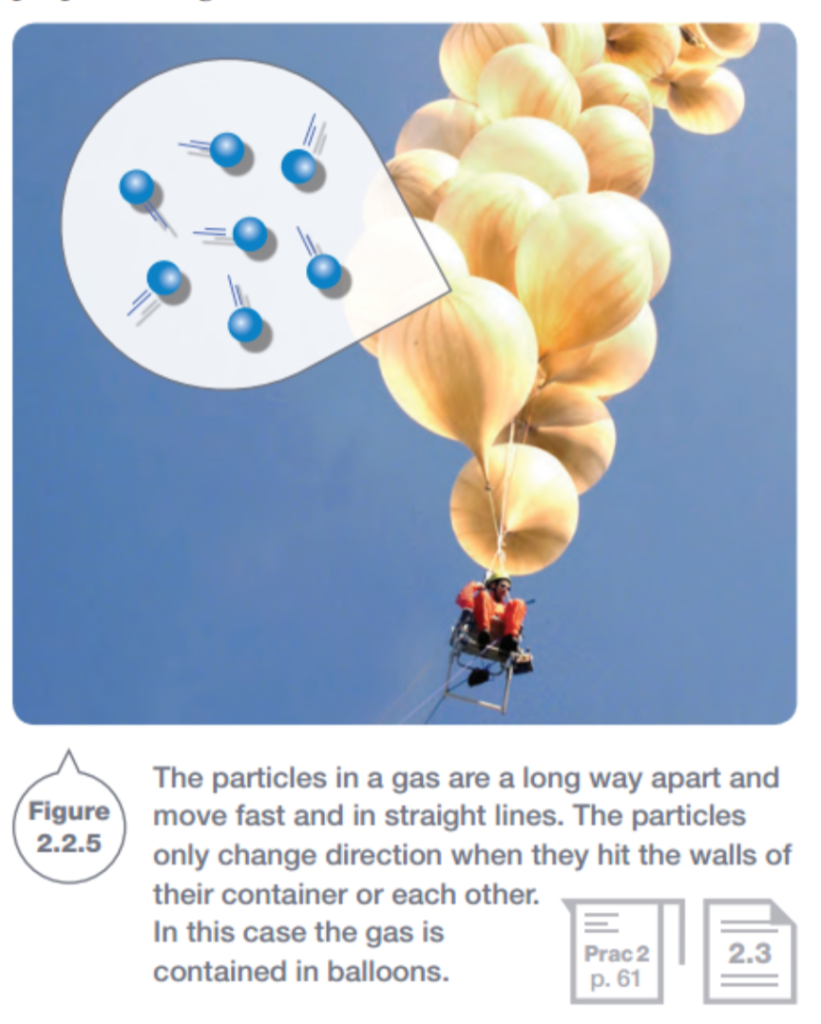
Gases have nothing holding their particles together. This lack of bonds allows the gas particles to travel randomly in straight lines until they hit something. The particles could hit other gas particles or the wall of the container they are in.

2.2 UNIT REVIEW

Answers:
-
Energy in the form of heat ✅
-
- 273˚C ✅
-
Solid – 3
Liquid – 2
Gas – 1
- a) Faster ✅
b) Slower ✅
- a) The particles for a solid are closely packed together and just jiggle in the spot. ✅
b) The particles for a liquid are packed closely together but are able to move about over one another this gives particles the ability to flow. ✅
c) The particles in a gas are a long way apart and move fast and in straight lines. The particles only change direction when they hit the walls of their container or each other. In this case, the gas is contained in balloons. ✅
- a) Vibrate – to move rhythmically and steadily ✅
b) Bond – a relationship between people or groups based on shared feelings, interests, or experiences.
-
?

