When you have heartburn taking an antacid tablet brings relief because of a chemical reaction between the acid in your stomach and bases within the tablet. The acid and base neutralize each other, forming harmless salts and water. If the acid had reacted with metal instead, then hydrogen gas would have formed. With carbonate, it would have produced carbon dioxide.
Acid and metals
Acids can corrode metals, usually forming salt and hydrogen gas.

The general equation for the reaction between an acid and a metal is:
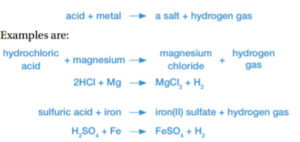
- Salt is normally the term used for Sodium Chloride (NaCl)
- However salt means any compound formed by a metal taking the place of a hydrogen atom in an acid
- For example, potassium nitrate (KNO3) is salt because potassium (a metal) has taken the place of hydrogen in nitric acid (HNO3).
- Likewise, magnesium sulfate (MgSO4) and calcium chloride (CaCl2) are salts because metals (Mg and Ca) have replaced hydrogen in sulfuric acid (H2SO4)
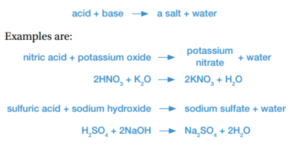
Neutralisation reactions
Acids and bases neutralize each other when mixed, changing each other into harmless substances such as water and salt. Neutralization reactions take the form:
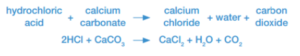
Acids and carbonates
When the base is a carbonate, the reaction between it and acid produces carbon dioxide as well as salt and water.
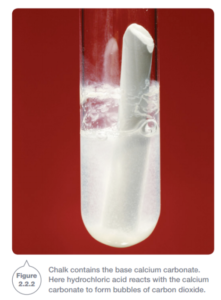
Carbon dioxide is also produced when acids react with hydrogen carbonates. For example, vinegar (ethanoic or acetic acid) and baking soda (sodium hydrogen carbonate) mix and form a froth of carbon dioxide bubbles.


Acid reactions indigestion
Chemical digestion is the process that breaks down large molecules in food into smaller ones that can be absorbed by the body. Chemical digestion is done mainly through a series of chemical reactions, each reaction being sped up by helper chemicals called enzymes. Enzymes in saliva help digest starch, enzymes in your stomach help digest protein, and enzymes in your duodenum help digest fat and any remaining carbohydrates and protein.
-
Enzymes need a specific, temperature and pH in order to function properly.
-
An enzyme called pepsin helps digest proteins in your stomach. Pepsin needs a strongly acidic environment to form and to work.
-
Gastric juice is produced by the lining of your stomach. It contains hydrochloric acid (HCl). This makes the stomach have a pH between 0 and 3.



